From the Journal: Applied Physics Reviews
WASHINGTON, May 5, 2021 – 3D bioprinting can create engineered scaffolds that mimic natural tissue. Controlling the cellular organization within those engineered scaffolds for regenerative applications is a complex and challenging process.
Cell tissues tend to be highly ordered in terms of spatial distribution and alignment, so bioengineered cellular scaffolds for tissue engineering applications must closely resemble this orientation to be able to perform like natural tissue.
In Applied Physics Reviews, from AIP Publishing, an international research team describes its approach for directing cell orientation within deposited hydrogel fibers via a method called multicompartmental bioprinting.
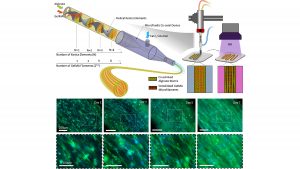
The team uses static mixing to fabricate striated hydrogel fibers formed from packed microfilaments of different hydrogels. In this structure, some compartments provide a favorable environment for cell proliferation, while others act as morphological cues directing cell alignment. The millimeter-scale printed fiber with the microscale topology can rapidly organize the cells toward faster maturation of the engineered tissue.
“This strategy works on two principles,” said Ali Tamayol, coauthor and an associate professor in biological engineering at UConn Health. “The formation of topographies is based on the design of fluid within nozzles and controlled mixing of two separate precursors. After crosslinking, the interfaces of the two materials serve as 3D surfaces to provide topographical cues to cells encapsulated within the cell permissive compartment.”
Extrusion-based bioprinting is the most widely used bioprinting method. In extrusion-based bioprinting, the printed fibers are typically several hundreds of micrometers in size with randomly oriented cells, so a technique providing topographical cues to the cells within these fibers to direct their organization is highly desirable.
Conventional extrusion bioprinting also suffers from high shear stress applied to the cells during the extrusion of fine filaments. But the fine scale features of the proposed technique are passive and do not compromise other parameters of the printing process.
To direct cellular organization, according to the team, extrusion-based 3D-bioprinted scaffolds should be made from very fine filaments.
“It makes the process challenging and limits its biocompatibility and the number of materials that can be used, but with this strategy larger filaments can still direct cellular organization,” said Tamayol.
This bioprinting technique “enables production of tissue structures’ morphological features — with a resolution up to sizes comparable to the cells’ dimension — to control cellular behavior and form biomimetic structures,” Tamayol said. “And it shows great potential for engineering fibrillar tissues such as skeletal muscles, tendons, and ligaments.”
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Controlling cellular organization in bioprinting through designed 3D microcompartmentalization
Authors
Mohamadmahdi Samandari, Fatemeh Alipanah, Keivan Majidzadeh-A, Mario M. Alvarez, Grissel Trujillo-de Santiago, and Ali Tamayol
Author Affiliations
University of Connecticut, Motamed Cancer Institute, Isfahan University of Medical Sciences, Tecnologico de Monterrey
