From the Journal: APL Bioengineering
WASHINGTON, December 1, 2020 — Until a vaccine is available, curbing the coronavirus pandemic relies heavily on how quickly a potentially exposed individual can be tested and quarantined. However, the current diagnostic techniques vary in reliability and relevance, so an understanding of which test is most appropriate for a given circumstance is necessary to avoid false reports.
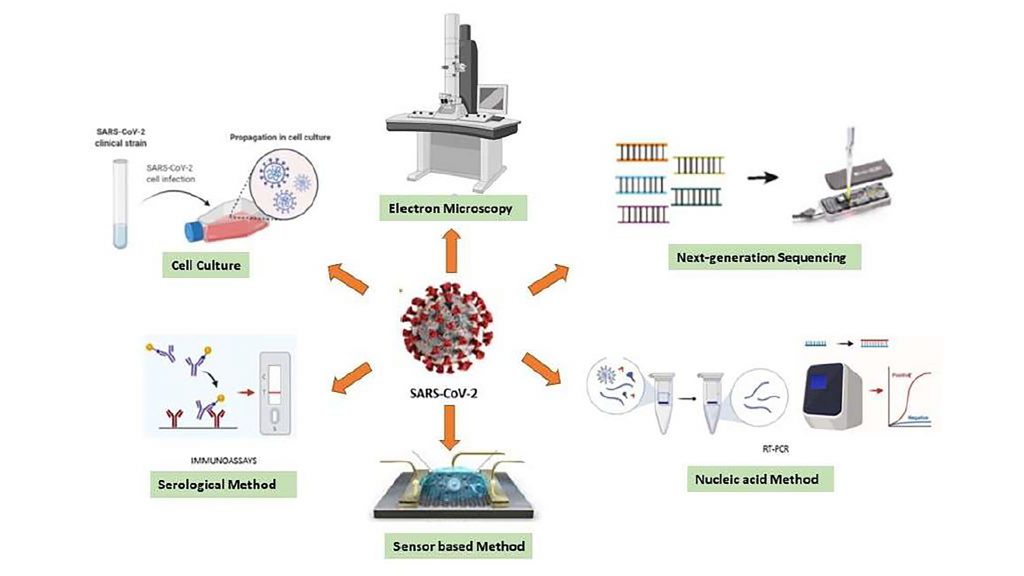
Researchers from Van Yuzuncu Yil University, Marmara University, Yildiz Technical University, and Istanbul Yeni Yuzyil University evaluated the available diagnostic techniques and determined key steps required for better testing moving forward. They present their findings in the journal APL Bioengineering, from AIP Publishing.
“Rapid diagnosis and rapid isolation are the key factors for prevention of the pandemic,” said Oguzhan Gunduz, one of the authors.
Laboratory tests that target the virus’s genes — known as real-time reverse transcription-polymerase chain reaction assays — are currently the gold standard for testing. But according to the Food and Drug Administration, these could give false negatives.
These tests depend on the presence of antibodies, which may not have yet been developed in the early stages of infection. Since different antibodies appear at different stages, diagnostic tests must be chosen to target the appropriate immune response based on when an individual is believed to have been infected.
“There is not any available single test for the entire stage of the disease,” Gunduz said. “However, I think it may be possible to detect the attack at any stage of the disease with nano-based sensor technologies.”
The group emphasizes point-of-care testing as an urgent objective. These types of tests would help detect the virus on site without the need for laboratory equipment or specialized personnel, eliminating or reducing the wait time between testing and obtaining results.
“A quite sensitive test that can measure the existent tiny number of viral particles, or any parameter related to the particle — weight, structure, charge, diameter — can provide rapid and early diagnosis,” said Gunduz.
When asked about the potential for a more comfortable testing method, he stressed that this depends on the sampling method and its sensitivity. A fingertip blood test or a saliva test could potentially be underway, though these have their own drawbacks.
“There are such tests, but they come up with accuracy and specificity issues,” Gunduz said.
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Evaluation of current diagnostic methods for COVID-19
Authors
Saadet Alpdagtas, Elif Ilhan, Ebru Uysal, Mustafa Sengor, Cem Bülent Üstündağ and Oguzhan Gunduz
Author Affiliations
Van Yuzuncu Yil University, Marmara University, Yildiz Technical University, and Istanbul Yeni Yuzyil University
