New method takes 3-D images of molecules in action
From the Journal: The Journal of Chemical Physics
WASHINGTON, D.C., April 4, 2017 — Quantum mechanics rules. It dictates how particles and forces interact, and thus how atoms and molecules work — for example, what happens when a molecule goes from a higher-energy state to a lower-energy one. But beyond the simplest molecules, the details become very complex.
“Quantum mechanics describes how all this stuff works,” said Paul Hockett of the National Research Council of Canada. “But as soon as you go beyond the two-body problem, you can’t solve the equations.” So, physicists must rely on computer simulations and experiments.
Now, he and an international team of researchers from Canada, the U.K. and Germany have developed a new experimental technique to take 3-D images of molecules in action. This tool, he said, can help scientists better understand the quantum mechanics underlying bigger and more complex molecules.
The new method, described in The Journal of Chemical Physics, from AIP Publishing, combines two technologies. The first is a camera developed at Oxford University, called the Pixel-Imaging Mass Spectrometry (PImMS) camera. The second is a femtosecond vacuum ultraviolet light source built at the NRC femtolabs in Ottawa.
Mass spectrometry is a method used to identify unknown compounds and to probe the structure of molecules. In most types of mass spectrometry, a molecule is fragmented into atoms and smaller molecules that are then separated by molecular weight. In time-of-flight mass spectrometry, for example, an electric field accelerates the fragmented molecule. The speed of those fragments depends on their mass and charge, so to weigh them, you measure how long it takes for them to hit the detector.
Most conventional imaging detectors, however, can’t discern exactly when one particular particle hits. To measure timing, researchers must use methods that effectively act as shutters, which let particles through over a short time period. Knowing when the shutter is open gives the time-of-flight information. But this method can only measure particles of the same mass, corresponding to the short time the shutter is open.
The PImMS camera, on the other hand, can measure particles of multiple masses all at once. Each pixel of the camera’s detector can time when a particle strikes it. That timing information produces a three-dimensional map of the particles’ velocities, providing a detailed 3-D image of the fragmentation pattern of the molecule.
To probe molecules, the researchers used this camera with a femtosecond vacuum ultraviolet laser. A laser pulse excites the molecule into a higher-energy state, and just as the molecule starts its quantum mechanical evolution — after a few dozen femtoseconds –another pulse is fired. The molecule absorbs a single photon, a process that causes it to fall apart. The PImMS camera then snaps a 3-D picture of the molecular debris.
By firing a laser pulse at later and later times at excited molecules, the researchers can use the PImMS camera to take snapshots of molecules at various stages while they fall into lower energy states. The result is a series of 3-D blow-by-blow images of a molecule changing states.
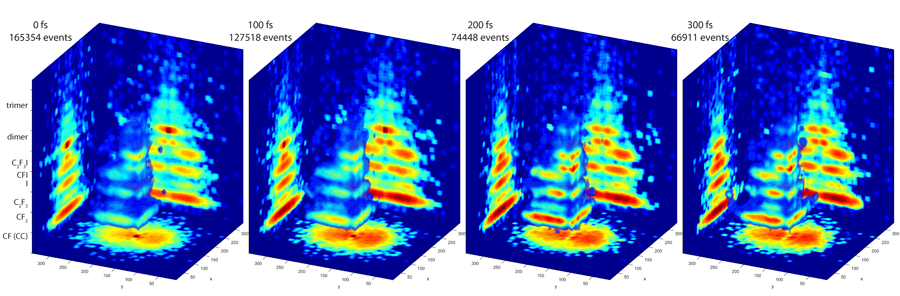
The researchers tested their approach on a molecule called C2F3I. Although a relatively small molecule, it fragmented into five different products in their experiments. The data and analysis software is available online as part of an open science initiative, and although the results are preliminary, Hockett said, the experiments demonstrate the power of this technique.
“It’s effectively an enabling technology to actually do these types of experiments at all,” Hockett said. It only takes a few hours to collect the kind of data that would take a few days using conventional methods, allowing for experiments with larger molecules that were previously impossible.
Then researchers can better answer questions like: How does quantum mechanics work in larger, more complex systems? How do excited molecules behave and how do they evolve?
“People have been trying to understand these things since the 1920s,” Hockett said. “It’s still a very open field of investigation, research, and debate because molecules are really complicated. We have to keep trying to understand them.”
###
For More Information:
Julia Majors
media@aip.org
301-209-3090
@AIPPhysicsNews
Article Title
Authors
Ruaridh Forbes, Varun Suresh Makhija, Kevin Veyrinas, Albert Stolow, Jason Lee, Michael B. Burt, Mark Brouard, Claire Vallance, Iain Wilkinson, Rune Lausten and Paul Hockett
Author Affiliations
University College London, University of Ottawa, University of Oxford, National Research Council of Canada and Helmholtz-Zentrum Berlin
