Using molecular dynamics simulations, researchers in New Mexico show that the shift in the glass transition temperature of a confined liquid (relative to a bulk liquid) can be approximately predicted.
From the Journal: The Journal of Chemical Physics
WASHINGTON, D.C., March 21, 2017 — Polymers are used for myriad applications today, and perhaps the most important property that dictates which polymer is chosen for a given application is its “glass transition temperature.” Many industrial polymers possess an irregular molecular structure that makes it impossible for them to crystallize. As a polymer material cools from a high temperature above its glass transition temperature, it morphs from a liquid to a glass when the transition temperature is reached.
While a polymer material has an amorphous, liquidlike structure in its glassy state, the mobility of the molecules is so low that they’re essentially frozen. So many hard plastics are, in fact, glassy. Polystyrene, for example, has a glass transition temperature of about 100 C — at room temperature it behaves like a solid material. But as its temperature approaches the glass transition temperature, polystyrene’s mechanical properties change drastically.
This makes the ability to approximate glass transitions for confined geometries in polymers highly desirable. And now, as a group of researchers from the University of New Mexico and New Mexico Institute of Mining and Technology reports in this week’s issue of The Journal of Chemical Physics, from AIP Publishing, they’ve developed a simple formula to do just that.
“With the development of nanotechnology, polymers have found many applications that require their use in ‘confined geometries’ such as narrow channels, small pores, and thin films,” explained the study’s co-author John Curro, an adjunct professor at New Mexico Institute of Mining and Technology
Over the past 20 years, experiments have shown that when polymers are used in a confined geometry, their glass transition “isn’t necessarily the same as for the corresponding ‘unconfined’ or bulk polymer,” Curro said. “It’s usually lowered, as is the case for free-standing films with two free surfaces, but it can also increase for liquids against strongly attracting substrates.”
The shift in glass transition depends sensitively on film thickness — the thinner the film the greater the effect. “This shift can be extraordinarily large,” Curro said. “For example, the glass transition temperature of a 20-nanometer polystyrene film has been measured as much as 70 C lower than bulk polystyrene. Clearly, this thin film of polystyrene is no longer a hard plastic material.”
As far as potential applications, “the fact that polymer properties in confined geometries are different than in bulk could have important implications for photolithography, nanocomposites, micromachines, and lab-on-a-chip devices,” Curro said.
So why is the glass transition of a confined polymer different than that of its corresponding bulk material?
“We hypothesized that it’s due to a density effect,” Curro said. “In an unconfined bulk liquid, the density is constant throughout the sample. By contrast, a confined liquid’s density of molecules is nonuniform because of constraints imposed by geometry.”
A freestanding film’s density, for example, is essentially zero at the two surfaces but increases to near the bulk density within the center. “Since the glass transition temperature depends strongly on density, we expect the local glass transition temperature to likewise vary throughout the film,” Curro said. “In a lab experiment, the measured glass transition temperature represents the average response of the material within the film. The average density of a freestanding film is generally different than its bulk density, and it follows that the glass transition temperatures will also be different.”
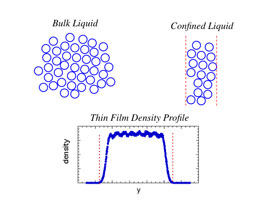 So the group explored whether the glass transition temperature of a confined liquid would be the same as a hypothetical bulk polymer — not at its normal bulk density, but rather at a density equal to the average density of the confined polymer.
So the group explored whether the glass transition temperature of a confined liquid would be the same as a hypothetical bulk polymer — not at its normal bulk density, but rather at a density equal to the average density of the confined polymer.
To put it to the test, they wanted to measure both the density profile and glass temperature on the same thin film. Such measurements would be difficult to carry out in the laboratory.
“Our approach was to use ‘molecular dynamics’ computer simulations to study thin liquid films consisting of short chain molecules,” Curro said. “We also performed computer simulations of the corresponding bulk system. This allowed us to compare the glass transition temperatures of thin films of various thicknesses with the bulk glass transition temperature on the same model chains.”
For computational efficiency, the group used an idealized bead-spring model of 10 beads to represent the molecules. By doing so, they “established a connection between the glass transition temperatures of a polymer in a constrained geometry and the corresponding bulk polymer,” Curro said. “This allowed us to develop a simple formula to estimate the glass transition of a confined liquid from the bulk glass transition temperature and a knowledge of the density profile of the confined system.”
It’s also important to note that the group’s results only apply to low molecular weight polymers and small molecule glasses.
“Subtle molecular weight effects are observed experimentally at high molecular weights when the average chain size is comparable to the film thickness, so high molecular weight will be a topic for future investigations,” Curro said.
###
For More Information:
Julia Majors
media@aip.org
301-209-3090
@AIPPhysicsNews
Article Title
The glass transition temperature of thin films: A molecular dynamics study for a bead-spring model
Authors
Craig Stevenson, John G. Curro and John D. McCoy
Author Affiliations
University of New Mexico, and the New Mexico Institute of Mining and Technology
