From the Journal: APL Bioengineering
WASHINGTON, Tuesday, April 28, 2020 — Biological robots, or biobots, draw inspiration from natural systems to mimic the motions of organisms, such as swimming or jumping. Improvements to biobots to better replicate complex motor behaviors can lead to exciting biorobotic engineering applications to help solve real world challenges. However, this requires the creation of biohybrid robots — biobots made up of both organic and artificial materials — which is a challenge.
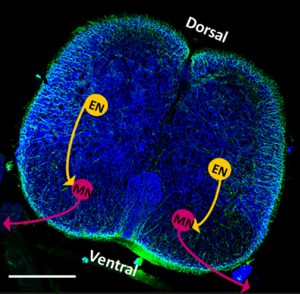
Researchers from the University of Illinois at Urbana-Champaign combined an intact rat spinal cord with a tissue-engineered, 3D muscle system. They describe the novel biohybrid system in the journal APL Bioengineering, from AIP Publishing.
After culturing the system for seven days, the researchers found that the motor neurons from the spinal cord begin to produce electrical activity that causes contraction in the artificial muscles, mirroring the behavior of the peripheral nervous system.
“When we looked more deeply at how the neuron-muscle interface developed, we were very excited to observe many similarities between our tissue-engineered spinobot and in vivo development,” said author Collin Kaufman, a neuroscience graduate student at UIUC.
Kaufman said this result indicates the explanted spinal cord is a viable mechanism for controlling muscular behaviors, even when removed from its natural environment.
They further tested this by varying the concentration of neurotransmitters in the system. When additional neurotransmitters are present, the contractions are more patterned and consistent, and when they are blocked, the twitching decreases.
Because studying the peripheral nervous system can be so difficult, the ability to observe it externally — as demonstrated in the present study — can lead to great strides in medicine.
One potential example is in Lou Gehrig’s disease, also known as amyotrophic lateral sclerosis, in which the death of neurons results in the eventual loss of motor function. By developing an external peripheral nervous system, researchers can study ALS with ease of access to the impacted components in real time.
“The next steps to studying such a disease are surprisingly close,” Kaufman said. “By replacing the muscle, the spinal cord, or any combination of the two tissues with an ALS mutant model, researchers would be able to study how diseased neurons interact with nearby muscles.”
In addition, hybrid biobots can be used as surgical training tools, allowing medical students to perform practice surgery on real biological tissue.
“The future applications of this technology are only beginning to be understood, and we expect many great things from this area in the next few years,” said Martha Gillette, professor of cell and developmental biology at UIUC.
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Authors
C.D. Kaufman, S.C. Liu, C. Cvetkovic, C. Lee, G. Naseri Kouzehgarani, R. Gillette, R. Bashir and M.U. Gillette
Author Affiliations
University of Illinois at Urbana-Champaign
