From the Journal: Biomicrofluidics
WASHINGTON, D.C., August 27, 2019 — Scientists are using nanoparticle screening on personal care products and finding previously thought toxic chemicals may not be harmful.
In a paper published in Biomicrofluidics, from AIP Publishing, researchers successfully used microchips to demonstrate titanium dioxide, a chemical found in most sunscreens, not only is nontoxic but also offers protection against ultraviolet damage to skin cells.
Because of their miniscule size, nanoparticles of a material don’t have the same physical, chemical, mechanical or optical properties as the same material would on a bigger scale. This means that something that might not be toxic on a bigger scale could be toxic on a smaller scale and vice versa.
Author Craig Priest said a nanomaterial included in a personal care product may remain on the body for many hours and be exposed to air, moisture, light and heat along with sweat, oils and wax from the skin. This could either enhance or diminish toxicity.
The authors chose to investigate UV radiation for its simplicity and for its potential for inclusion in a multiparameter study between nanoparticles and external stressors, such as sunlight.
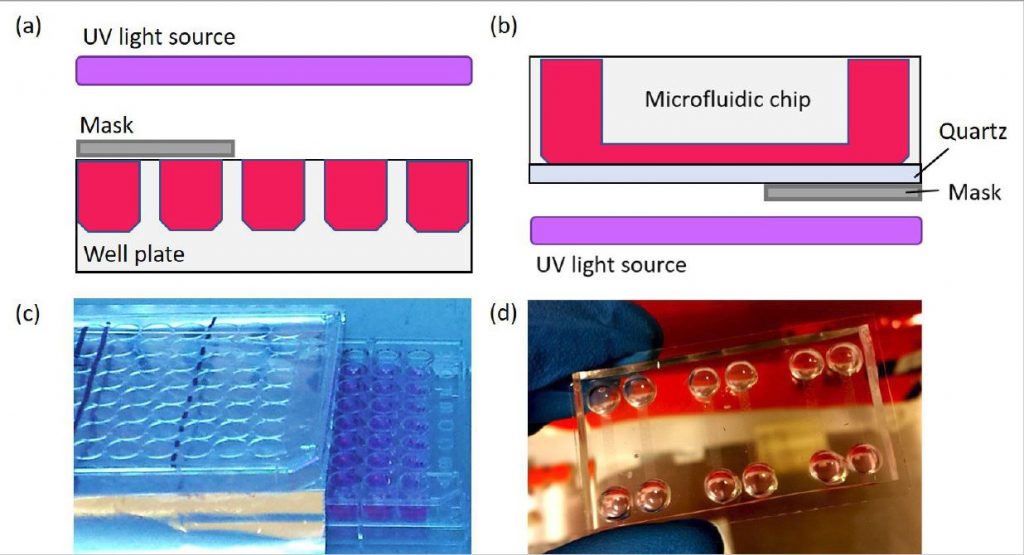
Previous nanotoxicity screening methods were laborious and time-consuming. Microfluidic devices present an optimistic future for nanoparticle analysis, offering reduced cost, small sample volumes, controllability and reproducibility.
“Nanoparticles are found in many products and vary greatly. The exact size, shape, material and surface properties determine whether a particle is safe or harmful, but the vast number of property combinations means that screening is extremely difficult,” Priest said.
The scientists built upon previous research, which tested microfluidic screening processes for chemicals, such as potassium cyanide, cycloheximide and chemotherapy drugs. A major advantage of using microfluidic devices is their ability to carry out multiple tests using small sample volumes in a compact microchip format.
“Our results will help speed the development of microfluidic screening chips and, in time, may form the basis of standardized screening of new nanoparticles before they enter products or the environment,” Priest said.
This research is expected to expedite toxicity testing of consumer personal products, as well as enhance safety and regulation on nanoparticles.
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Authors
Scott McCormick, Louise E. Smith, Amy Holmes, Ziqiu Tong, Enzo Lombi, Nicolas Voelcker and Craig Priest
Author Affiliations
University of South Australia and Monash University
