From the Journal: Biomicrofluidics
WASHINGTON, May 18, 2021 — What are the most delicate and valuable things you have handled? How would you feel if your daily job involved handling human eggs and any mistakes would affect someone’s life?
Typical egg collection requires a healthy woman to go through weeks of hormone therapy and then undergo an operation to retrieve eggs. These hard-earned and precious eggs are fertilized in vitro, and the best embryos are selected for future transfer.
But not all transfers succeed, which gives rise to the practice of freezing the extra embryos from an IVF cycle for future transfers. This allows those with at-risk fertility, due to age or treatments such as chemotherapy, to delay their transfer.
In the journal Biomicrofluidics, from AIP Publishing, researchers from the National Institute of Genetic Engineering and Biotechnology in Iran and McGill University and the University of British Columbia in Canada introduce a standalone microfluidics system to automate the process of embryo vitrification of replacing water with cryoprotectants.
Water is the enemy of any low-temperature system. Before embryos are frozen, the water inside must be replaced by cryoprotectants. But sudden removal of water kills embryos, so traditionally, embryos are transferred through multiple droplets with increasing concentrations of cryoprotectants. These transfers and washing steps added unnecessary pipetting steps.
“What if embryos simply stayed in the same place, and cryoprotectants were brought to them? Microfluidics systems are really good at controlling flow and concentration,” said Mojtaba Dashtizad, an assistant professor at the National Institute of Genetic Engineering and Biotechnology.
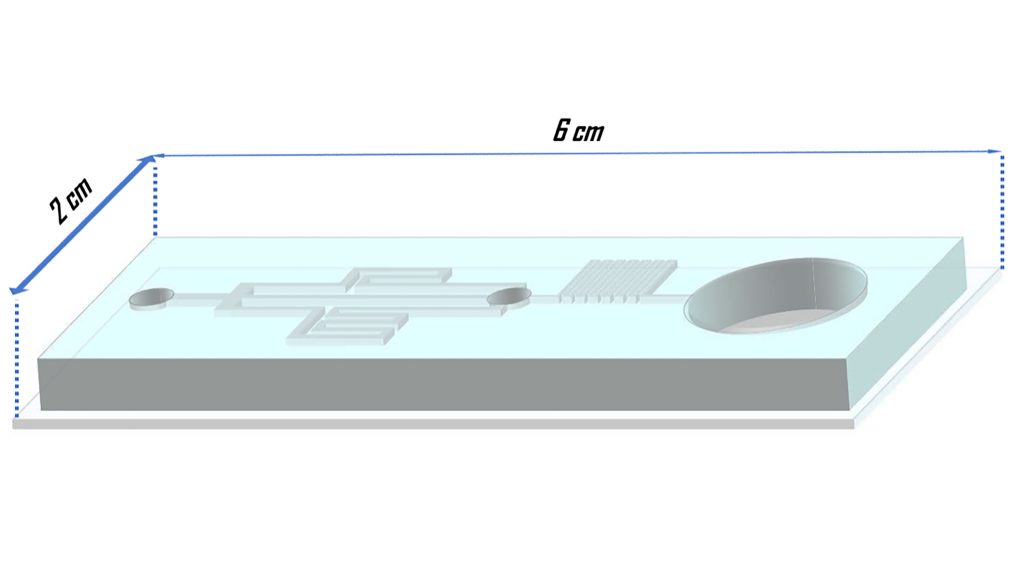
The researchers’ microfluidics setup has one chamber for an embryo to be placed, and three channels to gradually introduce cryoprotectants into it, while existing water is simultaneously emptied through an exit channel. Their single-inlet design of the chip simplifies technicians’ tasks to adding the cryoprotectant solution, placing the embryo, and waiting until the process is completed. The concentration of cryoprotectants is automatically adjusted by the chip.
Unlike the traditional method of cryopreservation, the new approach exposes embryos to a slow and constantly increasing concentration of cryoprotectants.
“Our genetic studies show this reduces molecular damage caused by cryopreservation,” said Dashtizad. “And embryos can be cryopreserved faster and with a lower concentration of cryoprotectants — a huge advantage because of the toxicity of these chemicals.”
This approach enables cryopreservation workflows to be simplified, more reproducible, and less prone to human error.
“Our findings emphasize the importance of moving away from droplet-based loading of cryoprotectants to gradual concentration controls,” said Dashtizad. “These can ultimately reduce damage to embryos during the cryoprocedure, and it moves us one step closer to increasing the efficiency of assisted reproduction and the improved health of future babies.”
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Authors
Pouria Tirgar, Fatemah Sarmadi, Mojgan Najafi, Parinaz Kazemi, Sina AzizMohseni, Samaneh Fayazi, Ghazaleh Zandi, Nikta Ziaie, Aida Shoushtari Zadeh Naseri, Allen Ehrlicher, and Mojtaba Dashtizad
Author Affiliations
National Institute of Genetic Engineering and Biotechnology in Iran, McGill University, University of British Columbia
