From the Journal: APL Bioengineering
WASHINGTON, January 19, 2021 — Biomedical engineers developed a technique to observe wound healing in real time, discovering a central role for cells known as fibroblasts. The work, reported in APL Bioengineering, by AIP Publishing, is the first demonstration of a wound closure model within human vascularized tissue in a petri dish.
Prior investigations of wound healing have used animal models, but healing in humans does not occur the same way. One difference is that wounds in mice and rats, for example, can heal without granulation tissue, a type of tissue critical to the healing of human wounds.
Granulation tissue forms after blood coagulates, and the wound scabs over. Coagulation creates a fibrin network that serves as a temporary matrix. Granulation tissue then takes over, filling the wound with new tissue and blood capillaries, protecting the wound from infection, and providing a foundation for further healing.
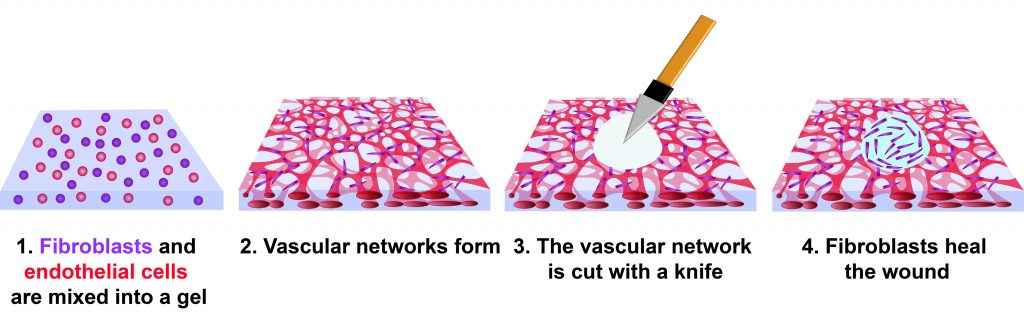
The investigators created a wound model by mixing human cells into a gel composed of fibrin and collagen. Blood vessels formed in the gel, producing what is known as vascularized tissue. Two cell types were used: human endothelial cells and fibroblasts, both of which are present in a natural wound.
Fibroblasts are natural fiber-producing cells found in most organs and connective tissue. Endothelial cells form the lining of blood vessels and regulate exchange between the bloodstream and other tissues. When a wound heals, new blood vessels form in a process known as angiogenesis.
Within three days, the mix of cells in the experimental gel had formed a network of capillaries. At this point, the tissues were cut with a diamond dissection knife, producing a wound through the full thickness of the tissue. It took four days for the wound to fully close, but cells were observed migrating into the wound by day 3.
“The migration of the fibroblasts and endothelial cells were tracked over the course of 90 hours, revealing rapid movement of fibroblasts around the wound edge and slower motion by endothelial cells,” said author Juliann Tefft.
The investigators observed fibroblasts circling the edge of the wound for about 50 hours, when the cells began to close the void. A series of subsequent experiments using varying amounts of fibroblasts and endothelial cells revealed that tissues with endothelial cells alone had not healed even after 10 days.
“This evidence supports the hypothesis that fibroblasts are the primary drivers of wound closure,” said author Jeroen Eyckmans. “In our system, the endothelial cells, needed for angiogenesis, the formation of new capillaries, didn’t repopulate the healed tissue that was filled in by the fibroblasts. For this reason, our model could be a useful bottom-up system to investigate the minimum components required for appropriate wound angiogenesis.”
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Reconstituting the dynamics of endothelial cells and fibroblasts in wound closure
Authors
Juliann B. Tefft, Christopher S. Chen, and Jeroen Eyckmans
Author Affiliations
Boston University
