Advances in microfluidics manufacturing show potential for improved artificial placentas to help preterm infants.
From the Journal: Biomicrofluidics
WASHINGTON, D.C., July 3, 2018 — Respiratory distress syndrome is the second major cause of newborn mortality. Health care providers especially struggle to deliver oxygen to preterm newborns, who account for roughly one-tenth of all births in the U.S., because lungs are among the last organs to fully develop in the womb. A new microfluidics innovation shows hope to improve artificial placentas so preterm newborns can properly develop lungs following birth.
An international team of researchers demonstrated a new technique to construct microchannels with a more efficient gas exchange between infant blood and air. The improved design uses both sides of the membrane for gas exchange and can potentially deliver nearly a third of the oxygen preterm newborns need without an external pumping mechanism. The group used this design to develop a prototype that oxygenates blood through a thin membrane. They report their findings in Biomicrofluidics, by AIP Publishing.
“The key innovation here is developing a large-area microfluidic device,” said P. Ravi Selvaganapathy, an author on the paper. “You want it to be microfluidic because a 1-kilogram baby, for example, might only have 100 milliliters of blood. You want a device to use only one-tenth of that volume at a time.”
Selvaganapathy and his team looked to the newborns’ own hearts to pump their device. This feature makes future oxygenators especially practical in areas with inconsistent electricity. After a child is born, his or her umbilical cord would be connected to an oxygenator. As the heart beats, it circulates blood through the umbilical cord, where it receives oxygen from the outside air.
“Rather than taking something that works in adults and trying to adapt it for neonates [newborns], we asked how would these designs look if we started designing these devices from scratch for babies, and built them from the ground up,” he said.
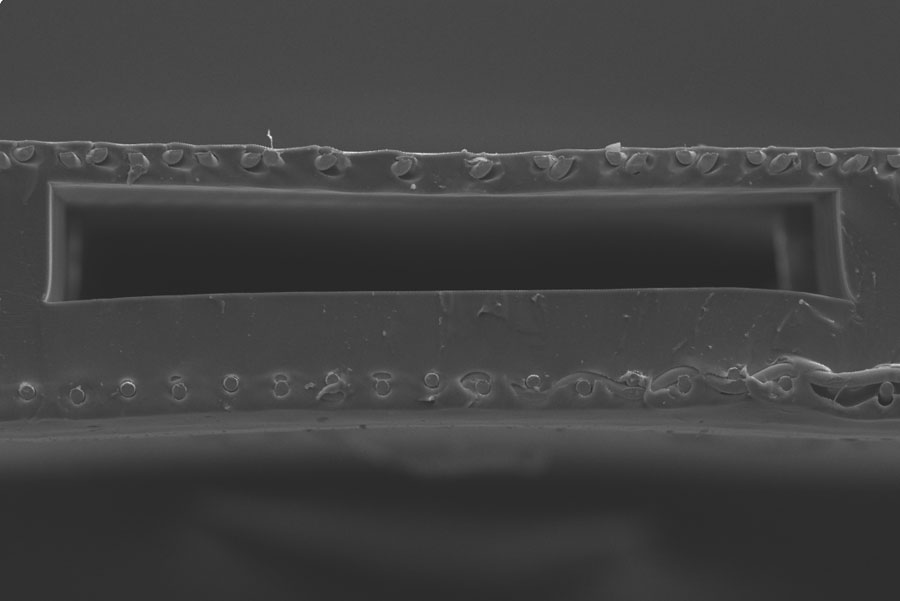
To achieve finely tuned control in the device, the researchers constructed a gas exchange membrane with a high surface area-volume ratio. Therefore, the device could mimic the placenta by ensuring the priming volume — how much blood is removed at a time for oxygenation — remained sufficiently low.
To do this, the team’s microfabrication methods allowed them to build a double-sided oxygenator that was reinforced by a 50-micron-thick stainless steel membrane surrounded by silicon rubber. The new oxygenator is more than three times as effective as their single-sided counterparts.
###
For More Information:
Julia Majors
media@aip.org
301-209-3090
@AIPPhysicsNews
Article Title
Authors
Mohammadhossein Dabaghi, Gerhard Fusch, Neda Saraei, Niels Rochow, John L. Brash, Christoph Fusch and P. Ravi Selvaganapathy
Author Affiliations
McMaster University
