From the Journal: APL Bioengineering
WASHINGTON, March 2, 2021 — Cardiovascular disease remains the number one cause of death globally. Unfortunately, the heart cannot regenerate new tissue, because the cardiomyocytes, or heart muscle cells, do not divide after birth.
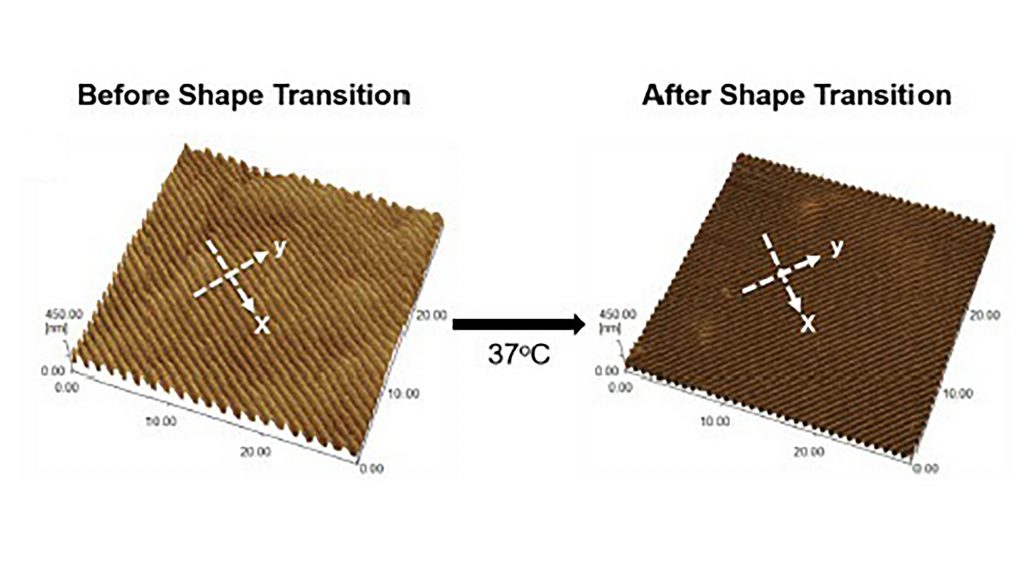
In their paper, published in APL Bioengineering by AIP Publishing, Syracuse researchers developed a shape memory polymer to grow cardiomyocytes. Raising the material’s temperature from 30 degrees Celsius to 37 degrees Celsius turned the polymer’s flat surface into nanowrinkles, which promoted cardiomyocyte alignment.
The research is part of the growing field of mechanobiology, which investigates how physical forces between cells and changes in their mechanical properties contribute to development, cell differentiation, physiology, and disease.
Scientists have developed cardiac microenvironments by incorporating external stimulations, such as pressure or stretching, to promote cardiomyocyte growth and maturation. But, they haven’t been able to control these microenvironments enough to reproduce the step-by-step gradual changes that occur in the body to understand the processes of rebuilding or remodeling heart tissue.
To address this challenge, researchers are using SRBs to learn more about how the microenvironment operates during heart development. SRBs, which are highly tunable, respond to temperature, pressure, electricity, and other external stimuli to provide cues for cell and tissue growth. SRBs undergo property switches in response to external stimuli, which means they can deliver on-demand changes that occur over time to affect the behaviors of cultured cells.
The ideal situation researchers are striving for is the creation of a synthetic 3D SRB-based cell culture platform that can change its material properties to mimic the natural progression of heart development. The platform could also help them learn more about the chemical and physical properties that lead to heart disease.
“It is crucial to understand how time-dependent biophysical cues affect cells during tissue formation,” said author Zhen Ma. “However, conceptual models are still based largely on results from studies of static, 2D experimental platforms in which biophysical cues remain constant over time.”
Ma suggests there should be a sharper focus on SRB electrical properties to expand the understanding of cardiac responses to extracellular changes. Embedding carbon nanotubes to enhance the conductivity of different polymer scaffolds, for instance, has been shown to improve intercellular communication and cardiomyocyte growth.
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Stimuli-responsive biomaterials for cardiac tissue engineering and dynamic mechanobiology
Authors
Huaiyu Shi, Chenyan Wang, and Zhen Ma
Author Affiliations
Syracuse University
