From the Journal: Applied Physics Reviews
WASHINGTON, May 11, 2021 — When trauma, illness, or injury causes significant muscle loss, reconstructive procedures for bioengineering functional skeletal muscles can fall short, resulting in permanent impairments.
Finding a synergy in the importance of biochemical signals and topographical cues, researchers from Wake Forest Institute for Regenerative Medicine, Sungkyunkwan University, and Chonnam National University developed an efficient technique for muscle regeneration and functional restoration in injured rats. They describe results from the technique in the journal Applied Physics Reviews, from AIP Publishing.
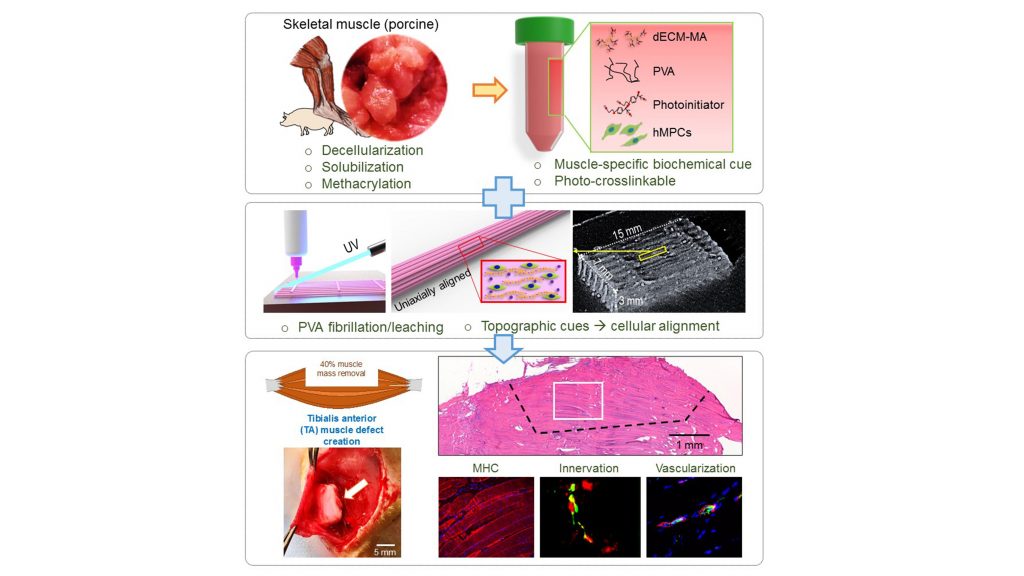
The group expanded on a method they previously developed using muscle-specific materials derived from an organism’s tissues (dECM-MA) to construct bioinks, which are materials used for 3D-printing tissue.
They combined dECM-MA from pigs’ skeletal muscles with pol(yvinyl alcohol) (PVA) fibrillation, a technique that provides cues to the molecules in the bioink to guide them to their target tissue and ensure they are properly aligned. By optimizing the PVA to enable stable, viable, well-aligned cell structures, the researchers were able to improve muscle regeneration and function restoration.
“One major benefit over previous approaches is the self-alignment of muscle cells in the 3D structure without any supporting components,” said Sang Jin Lee, one of the authors. “This could allow us to fabricate more clinically relevant bioengineered muscle constructs.”
They tested the technique on rats with injured muscles in their feet. When compared with uninjured rats of the same age and rats with the same injury but no treatment, the rats treated with the muscle regeneration technique showed over 80% muscle function restoration. Moreover, the bioengineered muscles integrated well with the rats’ neural and vascular systems.
These promising results suggest combining dECM-MA with PVA is a clinically feasible method for achieving large-scale tissue regeneration, provided the damage does not extend to nearby cells from which the tissue materials can be derived.
“This advanced bioprinting approach for bioengineering functional skeletal muscle constructs may be an effective therapeutic option for treating extensive muscle defect injuries with accelerated innervation and vascularization,” Lee said.
Since the technique requires cells from the patient, the group anticipates some hurdles in translating the methodology for human subjects, where it will be especially beneficial for systems that require cellular-level alignment, like cardiac and skeletal muscles. In the meantime, they plan to continue preclinical trials on larger, more clinically relevant muscle constructs in larger animals, such as rabbits, dogs, and pigs.
###
For more information:
Larry Frum
media@aip.org
301-209-3090
Article Title
Authors
Hyeongjin Lee, WonJin Kim, JiUn Lee, Kyung Soon Park, James J. Yoo, Anthony Atala, Geun Hyung Kim, and Sang Jin Lee
Author Affiliations
Wake Forest School of Medicine, Sungkyunkwan University, Chonnam National University Bitgoeul Hospital
