From the Journal: APL Bioengineering
WASHINGTON, May 23, 2023 – Type 1 diabetes affects 46.3 million people worldwide, and the number of people affected increases by about 3% each year. It requires careful calculations of insulin needs and bothersome daily injections to avoid peripheral diseases caused by extremes of high or low blood sugar.
Automated insulin delivery systems, also called artificial pancreases, make diabetes management much less onerous for patients. These systems — with implanted insulin sensors, pumps that deliver insulin inside the body, associated insulin pump controllers, and increasingly sophisticated control algorithms — are rapidly advancing.
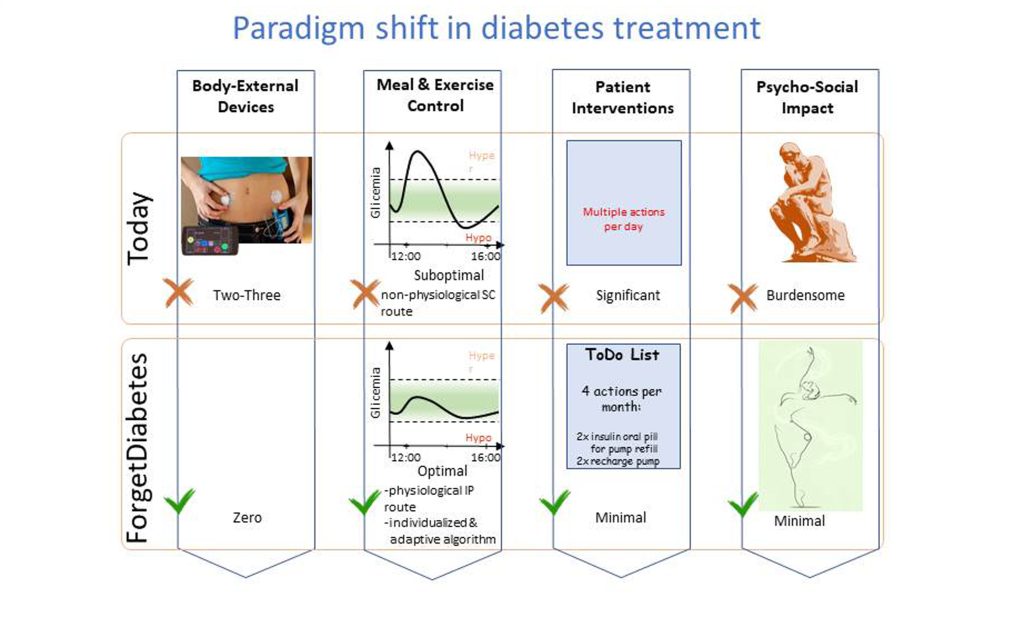
In APL Bioengineering, by AIP Publishing, researchers from the University of Padova, University of Pavia, and Yale University designed a novel algorithm for controlling implanted insulin pumps that accounts for the unique characteristics of individual patients. Their model, tested using an FDA-approved diabetes computer simulation, proves intraperitoneal (within the abdominal cavity) insulin delivery is fast and closely mimics natural physiological insulin delivery.
“Not only is intraperitoneal infusion of insulin much more physiological because you are reproducing the natural physiology, but it simplifies the control problem because you don’t have delays,” author Claudio Cobelli said. “So, this means you can have a very simple, robust controller to handle the everyday situations.”
The current method of automated insulin delivery, which is based on technology called continuous subcutaneous glucose sensors, requires patients to manually enter the number of carbohydrates they consume, announcing their meals to the system before they eat. It is also slow to sense and deliver insulin. These delays, along with the likelihood of errors in manual meal calculations, make the system prone to inaccuracies and increase the prevalence of hyperinsulinemia, a state of high insulin in patients that causes diseases of the large blood vessels.
Using an FDA-accepted simulator designed for continuous subcutaneous insulin delivery, the researchers made modifications to simulate intraperitoneal insulin delivery. They developed a model that can account for individual patient differences and validated a pump control algorithm that does not require meal announcement.
“This is a big plus. It helps with tuning the devices and allows personalization,” Cobelli said. “Different people have different needs, so you need to personalize the algorithms.”
Tying together previous work and current experiments, the researchers successfully showed the similarities between intraperitoneal insulin delivery and the physiology of natural insulin secretion and validated a pump control algorithm that is robust to personalization factors and time variance for breakfast, lunch, and dinner meals.
Their work is part of a multiyear, collaborative European project called “FORGET DIABETES” that aims to rapidly advance automated insulin delivery technologies to the point of clinical trials.
###
Article Title
Authors
Alberto Dalla Libera, Chiara Toffanin, Martina Drecogna, Alfonso Galderisi, Gianluigi Pillonetto, and Claudio Cobelli
Author Affiliations
University of Padova, University of Pavia, Yale University
